Abstract
Background: Granulocyte transfusions (GTX) in neutropenic patients (pts) have been used in an effort to treat infections not responding to antimicrobial therapy, but the efficacy and safety of GTX remains controversial. The objective of this study is to describe the outcome of pts with perirectal and perineal infections who received unirradiated GTX.
Methods: We conducted a retrospective review of pts with hematological diseases with perirectal or perineal infections who received GTX between 2014 and 2018. Clinical response of infection was considered at 7 days if there was improvement in imaging studies, successful de-escalation to oral antimicrobials, or documentation of resolution on physical exam. The pre and post white blood cell count (WBC), absolute neutrophil count (ANC), and platelets (PLT) after GTX was assessed. A hematological response was defined as ANC >0.5x109/L, and sustained hematological response as ANC >0.5x109/L 48 hours after GTX. Statistical analysis was performed with SPSS. Paired sample t tests were used to compare pre and post GTX counts, and independent sample t tests were used to compare median ANC response between categorical variables. Pearson's correlation coefficient was used to analyze continuous variables. Overall survival was estimated by Kaplan-Meier and compared with log-rank. Granulocyte donors were relatives/friends of the pts and were mobilized using G-CSF and dexamethasone prior to apheresis.
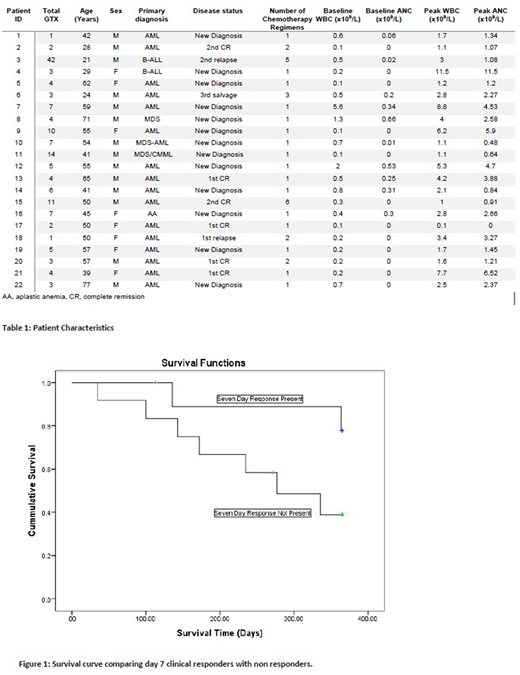
Results: 22 pts received a total of 148 GTX. Median number of GTX per patient was 4 (range 1-42). Median age of patient was 50 years (range 21-77). Seventeen pts had AML, 2 MDS, 2 ALL, and 1 had aplastic anemia. Six pts (27%) were in remission of their hematological disease at time of GTX. Median time from diagnosis of index infection to start of GTX was 7 days (range 1-24). Pts were receiving broad spectrum antibiotics including linezolid (9), meropenem (6), piperacillin tazobactam (5), ertapenem (4), and ceftazidime (4). Median number of antimicrobials per pt was 4 (range 2-5). Ten pts (45%) had clinical response of their infection at seven days post GTX. 20 pts (91%) achieved hematological response, all within 3 GTX, and 16 of them (73%) had sustained hematological response. All pts were able to resume chemotherapy. Median days from first GTX to next chemotherapy was 18.5. Difference between pre and post GTX WBC, ANC, and PLT were statistically significant (p<0.001). The median increase in WBC, ANC, and PLT was 0.20x109/L 0.26x109/L, and 3x109/L respectively. No association was found between gender (p=0.106), weight (p=0.169), age (p=0.616), number of GTX (p=0.133), or disease status (p=0.582) and median ANC increment. Day 7 clinical responders had a higher median ANC increment than those without response (1.349 vs 0.434, p=0.012), while no significant difference was found in mean number of GTX (p=0.073) or days to next chemotherapy (p=0.340). Disease status (p=0.069) or gender (p=0.746) did not predict day 7 response. There was no association between pts who held a 48-hr ANC response and day 7 response (p=0.489). Pts with sustained 48-hr response did not have a larger median ANC increment (p=0.068). Neither median ANC increment nor number of total GTX had a significant correlation with days to next chemotherapy (r -0.141 p=0.531, r 0.002 p=0.993). In 83 of the total 148 GTX (56%) ANC increased to >0.5x109/L. Day 7 responders did not have a higher percentage of successful transfusions than non-responders (p=0.236). All pts were alive 30 days after first GTX. 21 pts (96%) were alive at 90 days. With a median follow-up from the first GTX of 20.1 mo (3.77 to 40.6), 11 pts (50%) were still alive. Eleven pts have died, after a median of 33.57 weeks (14.29 to 161.57). There was trend for better 1 yr overall survival (OS) from time of first GTX for day 7 responders compared to non-responders (p=0.071). No serious adverse events were reported, including no transfusion association graft versus host disease, respiratory compromise, or anaphylaxis. Three pts had fever that was attributed to GTX and two had rash.
Conclusion: Unirradiated GTXs were tolerated without any significant complications and successfully raised ANC in all pts with perirectal or perianal infections. This can translate to cost effectiveness and better quality of life in the form of decreased days of inpatient intravenous antibiotics and shorter hospital stays. Further, prospective studies are warranted to conform these results.
Cortes:Pfizer: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Arog: Research Funding. Konopleva:Stemline Therapeutics: Research Funding. DiNardo:Karyopharm: Honoraria; Medimmune: Honoraria; Abbvie: Honoraria; Agios: Consultancy; Celgene: Honoraria; Bayer: Honoraria. Daver:Pfizer: Consultancy; Daiichi-Sankyo: Research Funding; Sunesis: Consultancy; ARIAD: Research Funding; Karyopharm: Research Funding; Novartis: Consultancy; Incyte: Consultancy; Sunesis: Research Funding; Kiromic: Research Funding; Novartis: Research Funding; Alexion: Consultancy; Incyte: Research Funding; BMS: Research Funding; ImmunoGen: Consultancy; Karyopharm: Consultancy; Otsuka: Consultancy; Pfizer: Research Funding. Pemmaraju:Affymetrix: Research Funding; SagerStrong Foundation: Research Funding; plexxikon: Research Funding; daiichi sankyo: Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; abbvie: Research Funding; cellectis: Research Funding; stemline: Consultancy, Honoraria, Research Funding; novartis: Research Funding. Kadia:Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Novartis: Consultancy; Takeda: Consultancy; BMS: Research Funding; Jazz: Consultancy, Research Funding; Celgene: Research Funding; Abbvie: Consultancy; Abbvie: Consultancy; Pfizer: Consultancy, Research Funding; Takeda: Consultancy; Amgen: Consultancy, Research Funding; BMS: Research Funding; Jazz: Consultancy, Research Funding; Celgene: Research Funding. Bose:Astellas Pharmaceuticals: Research Funding; Incyte Corporation: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; CTI BioPharma: Research Funding; Blueprint Medicines Corporation: Research Funding; Pfizer, Inc.: Research Funding; Constellation Pharmaceuticals: Research Funding. Andreeff:Amgen: Consultancy, Research Funding; Jazz Pharma: Consultancy; Eutropics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Daiichi-Sankyo: Consultancy, Patents & Royalties: MDM2 inhibitor activity patent, Research Funding; United Therapeutics: Patents & Royalties: GD2 inhibition in breast cancer ; Reata: Equity Ownership; SentiBio: Equity Ownership; Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Oncolyze: Equity Ownership; Aptose: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Ravandi:Xencor: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Abbvie: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Sunesis: Honoraria; Xencor: Research Funding; Sunesis: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria; Orsenix: Honoraria; Jazz: Honoraria; Abbvie: Research Funding; Jazz: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal